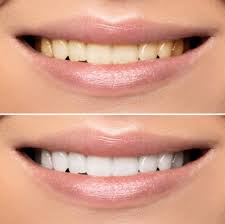
The process of teeth whitening lightens the color of teeth. Teeth whitening can be achieved by either changing the intrinsic color or by removing and controlling the formation of extrinsic stains. The chemical degradation of the chromogens within or on the tooth is termed as bleaching. Hydrogen peroxide (H2O2) is the active ingredient most commonly used in whitening products and is delivered as either hydrogen peroxide or carbamide peroxide.
Hydrogen peroxide is analogous to carbamide peroxide as it is released when the stable complex is in contact with water. When it diffuses into the tooth, hydrogen peroxide acts as an oxidizing agent that breaks down to produce unstable free radicals. In the spaces between the inorganic salts in tooth enamel, these unstable free radicals attach to organic pigment molecules resulting in small, less heavily pigmented components.
Reflecting less light, these smaller molecules create a “whitening effect”. There are different products available on the market to remove stains. For whitening treatment to be successful, dental professionals should correctly diagnose the type, intensity and location of the tooth discoloration. Time exposure and the concentration of the bleaching compound, determines the tooth whitening endpoint.
The natural shade of teeth
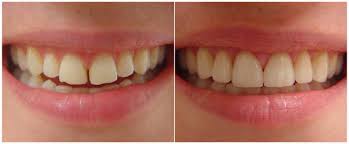
The perception of tooth color is multi-factorial. Reflection and absorption of light by the tooth can be influenced by a number of factors including specular transmission of light through the tooth; specular reflection at the surface; diffuse light reflection at the surface; absorption and scattering of light within the dental tissues; enamel mineral content; enamel thickness; dentine color, the human observer, the fatigue of the eye, the type of incident light, and the presence of extrinsic and intrinsic stains. Additionally, the perceived brightness of the tooth can change depending on the brightness and color of the background.
The combination of intrinsic color and the presence of extrinsic stains on the tooth surface influence the color and thus the overall appearance of teeth. The scattering of light and absorption within enamel and dentine determine the intrinsic color of teeth and because the enamel is relatively translucent, the dentinal properties can play a major role in determining the overall tooth color. On the other hand, extrinsic stain and color is the result of colored regions that have formed within the acquired pellicle on the enamel surface and can be influenced by lifestyle behaviours or habits. For example, dietary intake of tannin-rich foods, poor tooth brushing technique, tobacco products, and exposure to iron salts and chlorhexidine can affect the color of a tooth.
With increasing age, teeth tend to be darker in shade. This can be attributed to secondary dentin formation and thinning of enamel due to tooth wear which contributes to a significant decrease in lightness and increase in yellowness. Tooth shade is not influenced by gender or race.
Teeth discolouration and staining
Teeth discoloration and staining is primarily due to two sources of stain: intrinsic and extrinsic (see Figure 2). In essence, tooth whitening primarily targets those intrinsic stains in which cannot be removed through mechanics such as a debridement (clean) or prophylaxis, in the dental office. Below explains in-depth the differences between the two sources of which contribute to such discoloration of the tooth’s surface.
Environmental factors
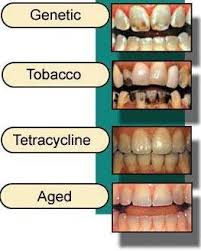
Environmental factors including smoking, pigments in beverages and foods, antibiotics, and metals such as iron or copper. Colored compounds from these sources are adsorbed into acquired dental pellicle or directly onto the surface of the tooth causing a stain to appear.
- Dental plaque: Dental plaque is a clear biofilm of bacteria that naturally forms in the mouth, particularly along the gum-line, and it occurs due to the normal development and defenses of the immune system. Although usually virtually invisible on the tooth surface, plaque may become stained by chromogenic bacteria such as Actinomyces species. Prolonged dental plaque accumulation on the tooth surface can lead to enamel demineralisation and formation of white spot lesions which appear as an opaque milk-colored lesion. The acidic by-products of fermentable carbohydrates derived from high-sugar foods contribute to greater proportions of bacteria, such as Streptococcus mutans and Lactobacillus in dental plaque. Higher consumption of fermentable carbohydrates will promote demineralisation and increase the risk of developing white spot lesions.
- Calculus: neglected plaque will eventually calcify, and lead to the formation of a hard deposit on the teeth, especially around the gum-line. The organic matrix of dental plaque and calcified tissues undergo a series of chemical and morphological changes that lead to calcification of the dental plaque and therefore leading to the formation of calculus. The color of calculus varies, and may be grey, yellow, black, or brown. The color of calculus depends on how long it has been present in the oral cavity for; it typically starts off yellow and over time the calculus will begin to stain a darker color and become more tenacious and difficult to remove.
- Tobacco: tar in the smoke from tobacco products (and also smokeless tobacco products) tends to form a yellow-brown-black stain around the necks of the teeth above the gum-line. The nicotine and tar in tobacco, combined with oxygen, turns yellow and over time will absorb into the pores of enamel and stain the teeth yellow. The dark brown to black stains along the gum line of the teeth are the result of the porous nature of calculus immediately picking up the stains from nicotine and tar.
- Betel chewing: Betel chewing produces blood-red saliva that stains the teeth red-brown to nearly black. The extract gel of betel leaf contain tannin, a chromogenic agent that causes discolouration of the tooth enamel.
Tannin is also present in coffee, tea, and red wine and produces a chromogenic agent that can discolor teeth. Large consumptions of tannin-containing beverages stain the dental enamel brown due to the chromogenic nature.
Certain foods, including curries and tomato-based sauces, can cause teeth staining.
Certain topical medications: Chlorhexidine (antiseptic mouthwash) binds to tannins, meaning that prolonged use in persons who consume coffee, tea or red wine is associated with extrinsic staining (i.e. removable staining) of teeth. Chlorhexidine mouthwash has a natural liking for sulphate and acidic groups commonly found in areas where plaque accumulates such as along the gum-line, on the dorsum of the tongue and cavities. Chlorhexidine is retained in these areas and stain yellow-brown. The stains are not permanent and can be removed with proper brushing.
Metallic compounds. Exposure to such metallic compounds may be in the form of medication or occupational exposure. Examples include iron (black stain), iodine (black), copper (green), nickel (green), and cadmium (yellow-brown). Sources of exposure to metal include placing metal into the oral cavity, metal-containing dust inhalation, or oral administration of drugs. Metals can enter the bony structure of the tooth, causing permanent discolouration, or can bind to the pellicle causing surface stain.
Extrinsic staining removal
 Extrinsic staining may be removed through various treatment methods:
Extrinsic staining may be removed through various treatment methods:
- Prophylaxis: dental prophylaxis includes the removal of extrinsic staining using a slow-speed rotary hand-piece and a rubber cup with abrasive paste, mostly containing fluoride. The abrasive nature of the prophy paste, as it is known, acts to remove extrinsic staining using the action of the slow-speed hand-piece and the paste against the tooth. Adversely, the action of the rubber cup together with the abrasive nature of the paste, removes around one micron of enamel from the tooth surface every time a prophylaxis is performed. This method of stain removal may only take place in the dental office.
- Toothpaste: there are many available on the market that implement both peroxide as well as abrasive particles such as silica gel to help remove extrinsic stains while the peroxide acts on intrinsic staining. This method of stain removal may take place at home as well as in a dental office.
- Micro-abrasion: allows a dental professional to make use of an instrument which emits a powder, water and compressed air to remove biofilm, and extrinsic staining. This stain removal method can only be undertaken in a dental office, not at home.
Intrinsic stains
Intrinsic staining primarily occurs during the tooth development either before birth or at early childhood. Intrinsic stains are those that cannot be removed through mechanical measures such as debridement or a prophylactic stain removal. As the age of the person increases, the teeth can also appear yellower over time. Below are examples of intrinsic sources of stains:
- Tooth wear and ageing: Tooth wear is a progressive loss of enamel and dentine due to tooth erosion, abrasion and attrition. As enamel wears down, dentine becomes more apparent and chromogenic agents are penetrated in the tooth more easily. The natural production of secondary dentine also gradually darkens teeth with age.
- Dental caries (tooth decay): The evidence regarding carious tooth discolouration is inconclusive, however the most reliable evidence suggests that carious lesion allows for exogenous agents to enter dentine and hence increased absorption of chromogenic agents causing discolouration to the tooth.
- Restorative materials: The materials used during root canal treatments, such as eugenol and phenolic compounds, contain pigment that stain dentine. Restorations using amalgam also penetrate dentine tubules with tin over time therefore causing dark stains to the tooth.
- Dental trauma which may cause staining either as a result of pulp necrosis or internal resorption. Alternatively the tooth may become darker without pulp necrosis.
- Enamel hypoplasia: Enamel hypoplasia causes enamel to be thin and weak. It produces a yellow-brown discolouration and can also cause the enamel’s smooth surface to be rough and pitted which causes the tooth to be susceptible to extrinsic staining, tooth sensitivity, malocclusion, and dental caries. The evidence regarding enamel hypoplasia is inconclusive, however the most likely cause is infection or trauma caused to the primary dentition. Disturbances to the developing tooth germ during neonatal and early childhood stages such as maternal vitamin D deficiency, infection, and medication intake can cause enamel hypoplasia.
- Pulpal hyperemia: Pulpal hyperemia refers to inflammation of a traumatized tooth which can be caused by a stimuli such as trauma, thermal shock, or dental caries. Pulpal hyperemia is reversible and produces a red hue seen initially after trauma which has the ability to disappear if the tooth becomes revascularized.
- Fluorosis: Dental fluorosis causes enamel to become opaque, chalky white, and porous. The enamel can break down and cause the exposed subsurface enamel to become mottled and produce extrinsic dark brown-black stains. Dental fluorosis occurs due to excessive ingestion of fluoride or overexposure to fluoride during the development of enamel which usually occurs between the ages of one to four. Fluoridated drinking water, fluoride supplements, topical fluoride (fluoride toothpastes), and formula prescribed for children can increase the risk of dental fluorosis. Fluoride is considered an important factor in the management and prevention of dental caries, the safe level for daily fluoride intake is 0.05 to 0.07 mg/kg/day.
- Dentinogenesis imperfecta: Dentinogenesis imperfecta is a hereditary dentine defect, associated with osteogenesis imperfecta, which causes the tooth to become discolored usually blue or brown in color and translucent giving teeth an opalescent sheen. The condition is autosomal dominant which means that the condition runs in the family.
- Amelogenesis imperfecta: The appearance of amelogenesis imperfecta depends on the type of amelogenesis, there are 14 different sub-types and can vary from the appearance of hypoplasia to hypomineralisation which can produce different appearances of enamel from white mottling to yellow brown appearances.
- Tetracycline and minocycline. Tetracycline is a broad-spectrum antibiotic, and its derivative minocycline is common in the treatment of acne. The drug is able to chelate calcium ions and is incorporated into teeth, cartilage, and bone. Ingestion during the years of tooth development causes yellow-green discoloration of dentine visible through the enamel which is fluorescent under ultraviolet light. Later, the tetracycline is oxidized and the staining becomes more brown and no longer fluoresces under UV light.
- Porphyria: A rare metabolic disorder in which the body fails to adequately metabolize porphyrins, which leads to accumulation or excretion of porphyrins into teeth. The excretion of porphyrins produces purple-red pigments in teeth.
- Hemolytic disease of the newborn: This disease occurs when a newborn’s red blood cells are being attacked by antibodies from the mother caused by an incompatibility between the mother and baby’s blood. This condition can produce green staining of teeth due to jaundice, which is an inability to excrete bilirubin properly.
- Root resorption: Root resorption is clinically asymptomatic, however can produce a pink appearance at the amelocemental junction.
- Alkaptonuria: Metabolic disorder which promotes the accumulation of homogentisic acid in the body and may cause brown color pigmentation in the teeth, gums, and buccal mucosa.
Procedures
 Prior to proceeding to tooth whitening alternatives, it is advised that the patient comes into the dental office to have a comprehensive oral examination that consists of a full medical, dental, and social history. This will allow the clinician to see if there is any treatment that needs to be done such as restorations to remove caries, and to assess whether or not the patient will be a good candidate to have the whitening done. The clinician would then debride (clean) the tooth surface with an ultrasonic scaler, hand instruments, and potentially a prophy paste to remove extrinsic stains as mentioned above. This will allow a clean surface for maximum benefits of whichever tooth whitening method the patient chooses. Below will discuss the various types of tooth whitening methods including both internal application of bleaching and external application through the use of bleaching agents.
Prior to proceeding to tooth whitening alternatives, it is advised that the patient comes into the dental office to have a comprehensive oral examination that consists of a full medical, dental, and social history. This will allow the clinician to see if there is any treatment that needs to be done such as restorations to remove caries, and to assess whether or not the patient will be a good candidate to have the whitening done. The clinician would then debride (clean) the tooth surface with an ultrasonic scaler, hand instruments, and potentially a prophy paste to remove extrinsic stains as mentioned above. This will allow a clean surface for maximum benefits of whichever tooth whitening method the patient chooses. Below will discuss the various types of tooth whitening methods including both internal application of bleaching and external application through the use of bleaching agents.
Treatment in the office

Before the treatment, the clinician should examine the patient: taking a health and dental history (including allergies and sensitivities), observe hard and soft tissues, placement and conditions of restorations, and sometimes x-rays to determine the nature and depth of possible irregularities. If this is not completed prior to the whitening agents being applied to the tooth surface, excessive sensitivity and other complications may occur.
The whitening shade guides are used to measure tooth color. These shades determine the effectiveness of the whitening procedure, which may vary from two to seven shades. These shades may be reached after a single in office appointment, or may take longer, depending on the individual. The effects of bleaching can last for several months, but may vary depending on the lifestyle of the patient. Consuming tooth staining foods or drinks that have a strong colour may compromise effectiveness of the treatment. These include food and drinks containing tannins such as; coffee, tea, red wines, and curry.
In-office bleaching procedures generally use a light-cured protective layer that is carefully painted on the gums and papilla (the tips of the gums between the teeth) to reduce the risk of chemical burns to the soft tissues. The bleaching agent is either carbamide peroxide, which breaks down in the mouth to form hydrogen peroxide, or hydrogen peroxide itself. The bleaching gel typically contains between 10% and 44% carbamide peroxide, which is roughly equivalent to a 3% to 16% hydrogen peroxide concentration. The legal percentage of hydrogen peroxide allowed to be given is 0.1–6%. Bleaching agents are only allowed to be given by dental practitioners, dental therapists, and dental hygienists.
Bleaching is least effective when the original tooth color is grayish and may require custom bleaching trays. Bleaching is most effective with yellow discolored teeth. If heavy staining or tetracycline damage is present on a patient’s teeth, and whitening is ineffective (tetracycline staining may require prolonged bleaching, as it takes longer for the bleach to reach the dentine layer), there are other methods of masking the stain. Bonding, which also masks tooth stains, is when a thin coating of composite material is applied to the front of a person’s teeth and then cured with a blue light. A veneer can also mask tooth discoloration.
In-chair whitening is faster and more effective in comparison to the take-home bleaching options. Some clinicians also make custom bleaching trays for you, which can take up to a week to create, so that after the whitening treatment is completed, you are able to use these trays in the future for maintenance of your bleaching with at home kits or for the use of desensitising products.
Power or light-accelerated bleaching
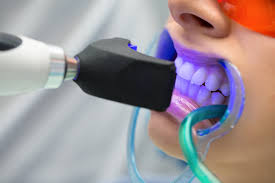 Power or light-accelerated bleaching uses light energy which is intended to accelerate the process of bleaching in a dental office. Different types of energy can be used in this procedure, with the most common being halogen, LED, or plasma arc. Use of light during bleaching increases the risk of tooth sensitivity and may not be any more effective than bleaching without light when high concentrations of hydrogen peroxide are used. A 2015 study showed that the use of a light activator does not improve bleaching, has no measurable effect, and rather is likely to increase the temperature of the associated tissues, resulting in damage.
Power or light-accelerated bleaching uses light energy which is intended to accelerate the process of bleaching in a dental office. Different types of energy can be used in this procedure, with the most common being halogen, LED, or plasma arc. Use of light during bleaching increases the risk of tooth sensitivity and may not be any more effective than bleaching without light when high concentrations of hydrogen peroxide are used. A 2015 study showed that the use of a light activator does not improve bleaching, has no measurable effect, and rather is likely to increase the temperature of the associated tissues, resulting in damage.
The ideal source of energy should be high energy to excite the peroxide molecules without overheating the pulp of the tooth. Lights are typically within the blue light spectrum as this has been found to contain the most effective wavelengths for initiating the hydrogen peroxide reaction. A power bleaching treatment typically involves isolation of soft tissue with a resin-based, light-curable barrier, application of a professional dental-grade hydrogen peroxide whitening gel (25–38% hydrogen peroxide), and exposure to the light source for 6–15 minutes. Recent technical advances have minimized heat and UV emissions, allowing for a shorter patient preparation procedure.
For any whitening treatments, it is recommended that a comprehensive examination of the patient is done including the use of radio-graphs to aid in the diagnosis of the current condition of the mouth, including any allergies that may be present. The patient will need to have a healthy mouth and free of periodontal disease or caries and to have had a debridement/clean done to remove any tartar or plaque build up.
It is recommended to avoid smoking, drinking red wine, eating or drinking any deeply colored foods after this as the teeth may stain considerably straight after treatment.
New light-accelerated bleaching agents

A recent addition to the field is new light-accelerated bleaching agents containing lower concentrations of hydrogen peroxide with a titanium oxide nano-particle-based catalyst. Reduced concentrations of hydrogen peroxide cause lower incidences of tooth hypersensitivity. The nano-particles act as photo-catalysts, and their size prevents them from diffusing deeply into the tooth. When exposed to light, the catalysts produce a rapid, localized breakdown of hydrogen peroxide into highly reactive radicals. Due to the extremely short lifetimes of the free radicals, they are able to produce bleaching effects similar to much higher concentration bleaching agents within the outer layers of the teeth where the nano-particle catalysts are located. This provides effective tooth whitening while reducing the required concentration of hydrogen peroxide and other reactive byproducts at the tooth pulp.
Bleaching Internally
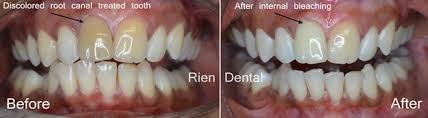 Internal bleaching is a process which occurs after a tooth has been endodontically treated. This means that the tooth will have had the nerve of the tooth extirpated or removed through a root canal treatment at the dentist or by a specialist endodontist. Internal bleaching is often sought after in teeth which have been endodontically treated as tooth discolouration becomes a problem due to the lack of nerve supply to that tooth. It is common to have this internal bleaching done on an anterior tooth (a front tooth that you can see when smiling and talking). A way around this is by sealing off the bleaching agent inside the tooth itself and replacing it every few weeks until the desired shade has been achieved. The amount of time between appointments varies from patient to patient and with operator preference until the desired shade has been achieved. Even though this is a great option, the disadvantage of this treatment is a risk of internal root resorption of the tooth that is being internally bleached. This may not occur in every patient or every tooth, and its occurrence is difficult to determine prior to completing the treatment.
Internal bleaching is a process which occurs after a tooth has been endodontically treated. This means that the tooth will have had the nerve of the tooth extirpated or removed through a root canal treatment at the dentist or by a specialist endodontist. Internal bleaching is often sought after in teeth which have been endodontically treated as tooth discolouration becomes a problem due to the lack of nerve supply to that tooth. It is common to have this internal bleaching done on an anterior tooth (a front tooth that you can see when smiling and talking). A way around this is by sealing off the bleaching agent inside the tooth itself and replacing it every few weeks until the desired shade has been achieved. The amount of time between appointments varies from patient to patient and with operator preference until the desired shade has been achieved. Even though this is a great option, the disadvantage of this treatment is a risk of internal root resorption of the tooth that is being internally bleached. This may not occur in every patient or every tooth, and its occurrence is difficult to determine prior to completing the treatment.
At home treatment
At home tooth whitening products are available from dentists or ‘over the counter’ (OTC). At home whitening methods include; over the counter strips and gels, whitening rinses, whitening toothpastes, and tray-based tooth whiteners. OTC products can be used for milder cases of tooth staining. Home-based bleaching (following manufacturer’s instructions) would result in less tooth sensitivity than in-office bleaching.
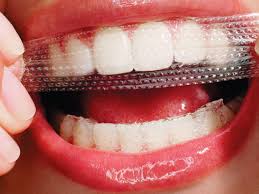 Strips and gels
Strips and gels
The plastic whitening strips contain a thin layer of peroxide gel and are shaped to fit the buccal/labial surfaces of teeth. Many different types of whitening strips are available on the market, after being introduced in the late 1980s. Specific whitening strip products have their own set of instructions however the strips are typically applied twice daily for 30 minutes for 14 days. In several days, tooth color can lighten by 1 or 2 shades. The tooth whitening endpoint does depend on the frequency of use and ingredients of the product.
Whitening gels are applied onto the tooth surface with a small brush. The gels contain peroxide and are recommended to be applied twice a day for 14 days. The tooth whitening endpoint like that of the whitening strips.
Rinses
Whitening rinses work by reaction of the oxygen sources such as hydrogen peroxide within the rinse and the chromogens on or within the tooth. It is recommended to use twice a day, rinsing for one minute. To see an improvement in shade color, it can take up to three months.
Toothpastes
 Whitening toothpastes are different to regular toothpastes in that they contain higher amounts of abrasives and detergents to be more effective at removing tougher stains. Some whitening toothpastes contain low concentrations of carbamide peroxide or hydrogen peroxide which help lighten tooth color however they do not contain bleach (sodium hypochlorite). With continuity of use over time, tooth color can lighten by one or two shades.
Whitening toothpastes are different to regular toothpastes in that they contain higher amounts of abrasives and detergents to be more effective at removing tougher stains. Some whitening toothpastes contain low concentrations of carbamide peroxide or hydrogen peroxide which help lighten tooth color however they do not contain bleach (sodium hypochlorite). With continuity of use over time, tooth color can lighten by one or two shades.
Tray-based
 Tray-based teeth whitening is achieved by wearing a fitted tray containing carbamide peroxide bleaching gel overnight or for two to four hours a day. If manufacturer’s instructions are followed, tooth whitening can occur within three days and lighten teeth by one or two shades. This type of tooth whitening is available over-the-counter and professionally from an oral health professional.
Tray-based teeth whitening is achieved by wearing a fitted tray containing carbamide peroxide bleaching gel overnight or for two to four hours a day. If manufacturer’s instructions are followed, tooth whitening can occur within three days and lighten teeth by one or two shades. This type of tooth whitening is available over-the-counter and professionally from an oral health professional.
Baking soda
 Baking soda is a safe, low abrasive, and effective stain removal and tooth whitening toothpaste. Tooth whitening toothpaste that have excessive abrasivity are harmful to dental tissue, therefore baking soda is a desirable alternative. To date, clinical studies on baking soda report that there have been no reported adverse effects. It also contains acid-buffering components that makes baking soda biologically antibacterial at high concentrations and capable of preventing growth of Streptococcus mutans. Baking soda might be useful for caries-prone patients as well as those who wish to have whiter teeth.
Baking soda is a safe, low abrasive, and effective stain removal and tooth whitening toothpaste. Tooth whitening toothpaste that have excessive abrasivity are harmful to dental tissue, therefore baking soda is a desirable alternative. To date, clinical studies on baking soda report that there have been no reported adverse effects. It also contains acid-buffering components that makes baking soda biologically antibacterial at high concentrations and capable of preventing growth of Streptococcus mutans. Baking soda might be useful for caries-prone patients as well as those who wish to have whiter teeth.
Oil-pulling
 Oil pulling is a traditional remedy originating from Ayurvedic medicine in India. Oil pulling is the practice of swishing oil (typically coconut, sesame or sunflower oil) around the mouth for up to 15 minutes per day and can be used as a natural approach to whiten teeth. Coconut oil attacks harmful bacteria (Streptococcus mutans and Candida albicans) that cause tooth decay. The lauric acid, a medium chain fatty acid, present in coconut oil, has proven anti-inflammatory and antimicrobial effects. Monolaurin has proven the ability to alter and penetrate microbial cell walls, cell membranes and inhibit enzymes linked to energy product and transfer of nutrients which results in bacterial death. The removal of bacteria leads to improved oral hygiene and, as a result, whiter teeth. This practice should be done in conjunction with a regular oral health regime. This is all in accordance with Wikipedia.
Oil pulling is a traditional remedy originating from Ayurvedic medicine in India. Oil pulling is the practice of swishing oil (typically coconut, sesame or sunflower oil) around the mouth for up to 15 minutes per day and can be used as a natural approach to whiten teeth. Coconut oil attacks harmful bacteria (Streptococcus mutans and Candida albicans) that cause tooth decay. The lauric acid, a medium chain fatty acid, present in coconut oil, has proven anti-inflammatory and antimicrobial effects. Monolaurin has proven the ability to alter and penetrate microbial cell walls, cell membranes and inhibit enzymes linked to energy product and transfer of nutrients which results in bacterial death. The removal of bacteria leads to improved oral hygiene and, as a result, whiter teeth. This practice should be done in conjunction with a regular oral health regime. This is all in accordance with Wikipedia.
All of these products can be found in JMJ45TECH’s ONLINE STORE. Thank you for your support.
Please Leave All Comments in the Comment Box Below ↓











This is a very in-depth and informative piece. Have you noticed how people are judged by how their teeth look? I have some serious bone loss due to genetics and smoking (periodontal disease). They have shifted around and are a jumbled mess because of it. Believe it or not, they were once naturally straight. So, as a result, I don’t smile much anymore.
Thanks,
Carrie
Hello Carrie,
Thank you for taking the time to read and comment on this post.
To answer your question, Yes, I have noticed how people are judged by how their teeth look. Honestly, at some point in my younger life, I was probably one of those people, and the nerve of me to have felt this way with the fact being, my teeth are very crooked.
I am sorry to hear about your periodontal disease.
Thank you again for your comment.
Many Blessings To You My Friend!
Hello it’s me again,
You have really jam packed this page with great information. I work in a dental office and my dentists swear by the in office whitening. I do not know if that is because it actually works or because it costs the patients more money.
Anywho, I have used whitening strips at home and those worked kind of well for me. Now they have the take home trays with whitening gel for people and that is pretty popular in our office too. The best recommendation from my dentists is to do the in office whitening but not too powerful at once because it can hurt your enamel. Then do the take home whitening gel after you do the in office whitening.
Thank you again for reading, commenting, and bringing up such relevant recommendations about another article I have created.
Thank you very much for all of your input on each article, As you are the day to day expert in this field, and it truly means a lot to me to learn that you consider there to be great information jammed packed in this article.
Blessings To You My Friend!
A very great article with lots of useful informations.
Teeth whitening is something that every individual has to have to keep a good hygiene.
Reading this article gave me lots of informations I absolutely had no idea about which I would share to others. I suggest everyone gets their teeth whitened since because having coloured teeth can sometimes be disgusting.
Great article
Thank you for taking the time to read, comment, and for considering this a great post, and thank you for considering it that. I think going into deep details helps to answer questions as the reader continues reading.
Thank you again for taking the time to read and comment on this post.
Best Of Blessings To You My Friend!